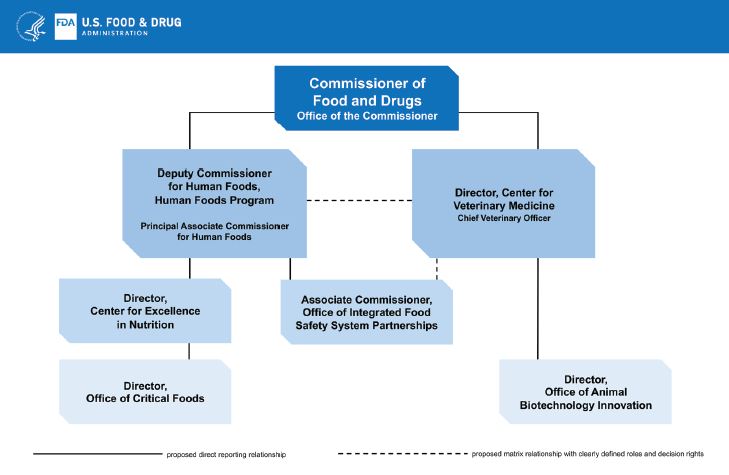
 The FDA reorg chart for the new Human Foods Program
The FDA reorg chart for the new Human Foods Program
Feb 01, 2023
By Dr. John Goldberg
Goldberg is the founder of Science Based Strategies, a Washington, DC-based food, agriculture, and environmental policy consulting firm, and a partner at the Normandy Group, LLC.
WASHINGTON, DC – Yesterday, Food and Drug Administration (FDA) Commissioner Dr. Robert Califf announced a reorganization within the U.S. Food and Drug Administration (FDA) where the functions of the Center for Food Safety and Applied Nutrition (CFSAN), Office of Food Policy and Response (OFPR), as well as certain functions of the Office of Regulatory Affairs (ORA) will be unified into a newly envisioned organization called the Human Foods Program under a Deputy Commissioner for Human Foods. The person in this position will report directly to the Commissioner.
“It is great to see the FDA taking action to follow the recommendations presented in the December 2022 Reagan-Udall Foundation report and address some of the issues identified with regard to food safety and nutrition programs,” said USA Rice President & CEO Betsy Ward.
The Foundation report suggested that the agency create a Deputy Commissioner for Foods with line authority over the Human Foods Program – a proposal supported by many in the food and agricultural community. “Through this restructuring, the domestic rice industry, as well as the greater food and beverage industry, will benefit from greater transparency on regulatory actions, a more streamlined and science-based process around such actions, and a structure that is focused on protecting a safe, nutritious food supply with the flexibility to adapt to changes,” said Ward.
Potential weaknesses in this plan include lack of clarity on budgetary authority of the new Deputy Commissioner for Human Foods, and concerns that reorganized functions of ORA will not include field investigation and inspection personnel. These functions will not report to the new Human Foods Program, thereby maintaining a separate leadership structure and the historical disconnect between field personnel and those in Washington who write the rules.
The food and agriculture community has been pressing for fundamental changes at FDA and just last week, FDA Deputy Commissioner Frank Yiannas resigned, citing flaws in the agency’s crisis response capabilities. In his resignation letter to letter to Commissioner Califf, Yiannas stated that the “decentralized structure of the foods program that you and I both inherited, significantly impaired FDA’s ability to operate as an integrated food team and protect the public.”
USA Rice works closely with FDA on various issues including nutrition, food labeling, and food safety, and most recently on FDA’s Closer-to-Zero Initiative involving reducing levels of heavy metals in food intended for infants and children.
“USA Rice has a positive, collaborative working relationship with FDA,” said Ward. “We believe that the reorganization announced this week by Commissioner Califf will further strengthen this relationship by designating a single individual in the agency who is accountable and accessible to the industry they’re regulating.”